Interface between Water–Solvent Mixtures and a Hydrophobic Surface
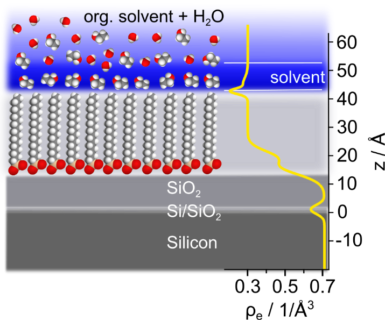
The mechanism behind the stability of organic nanoparticles prepared by liquid anti-solvent precipitation (LAS) without specific stabilizing agent is poorly understood. Therefore, we mimicked the solid-liquid interface of a hydrophobic organic nanoparticle with an OTS coated silicon wafer and studied the molecular structure of the interface between the OTS layer and a solution of an organic solvent in water with X-ray reflectometry.
We found that an organic solvent layer is formed at the interface with a thickness systematically increasing with the concentration of the solvent up to the length of 1 to 2 solvent molecules. This layer was observed for all organic solvents and increased with decreasing polarity of the solvent molecule. Finally, we demonstrate that the layer formation could be explained by a Langmuir adsorption model for acetone and THF and a BET isotherm for the alcohols studied. We developed a self-consistent adsorption model via complementing the adsorption isotherms obtained from XRR data with molecular dynamics (MD) simulations.
Read the full article in Langmuir